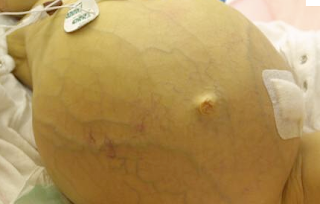
 ascites photo borrowed from internet
ascites photo borrowed from internet
This patient presented with alcoholic cirrhosis and a MELD score of 19. His management involves normalizing several aspects of his physiology. I would like to take the time to review the pathophysiology of cirrhosis as it applies to this case. The word cirrhosis derives from the greek kirrhos meaning tawny or brown/orange. This is because cirrhotic livers have a burnt brownish orange appearance. Physiologically, cirrhosis is an irreversible fibrosis of the liver whereby the cellular architecture is destroyed and unable to be regenerated. As a result dysfunctional nodular tissue forms in place of viable parenchyma, and this ensuing disordered tissue greatly increases intrahepatic vascular resistance. This resistance creates a pressure gradient between the portal vein and hepatic, the so-called hepatic vein portal gradient (HVPG). Because passage through portal circulation is greatly impeded over time, the HVPG increases. When the gradient reaches ~12mmHg, a diagnosis of portal hypertension can be made, although clinical symptoms are not usually apparent until this gradient is greater than 20mmHg1. The portal hypertension is necessary for the accumulation of fluid in the peritoneal cavity, ascites. Specifically, ascites comes as a direct result of this increased HVPG. A sustained HVPG over time develops porto-systemic collaterals through angiogenic mechanisms. These new shunts in addition to the known shunts (gastric vein, rectal vein, umbilical veins) greatly decrease systemic vascular resistance and increase cardiac output. Also the increased pressure in the portal system in concert with increased levels of bacterial endotoxin from decreased portal flow has been found to induce high levels of NO release from the endothelium. This NO release is the prime mechanism for the great splanchnic vasodilation found in cirrhotic patients with ascites. Thus the portal system becomes greatly dilated and “stopped up” while the systemic circulatory systemic becomes one of high flow and low resistance. This high flow system also reduces mean arterial pressure thus decreasing the carotid and renal baroreceptor “stretch” and activating the neurohumoral responses of the renin angiotensin-aldosterone activating system (RAAS), sympathetic activation and ADH. Chronic activation of these factors leads to sodium retention and an inability to excrete salt that can greatly derange volume status. Decreased urine sodium excretion (sometimes as little as 10meq/day) is commonly found in cirrhotics2. Chronically unopposed ADH action resulting from underperfusion of the carotid baroreceptors doubly impedes the body’s ability to regulate volume because the kidneys no longer can excrete free water (inhibited by ADH). Overtime a refractory dilutional hyponatremia results from this response. In fact with cirrhosis, the patient is volume and salt overloaded despite having a low intravascular volume and hyponatremia. Unfortunately, these mechanisms of maintaining volume ultimately fail because as the synthetic capability of the liver declines so too does the synthesis of a prime mediator of the intravascular volume, albumin. The decreasing oncotic effects of these proteins and increased splanchnic capillary dilation give are greatly hindered and this excess volume is transmitted to the interstitial spaces and potential spaces of the peritoneum. Finally because the intravascular volume cannot be maintained, this cycle continues unbroken.
Ascites in liver cirrhosis is but one of many medical problems that this patient faces. There is a risk of the peritoneal fluid becoming septic, so called spontaneous bacterial peritonitis (SBP). This is associated with great mortality and requires chronic antibiotic prophylaxis. Particularly relevant to this patient is the risk of catastrophic bleeding from portosystemic varices that evolve as a result of increased portal pressures and high flow portosystemic shunting. It is presumed that this likely contributed in significant ways to creating his evolving anemia, and although he was not found to have active bleeding on EGD, his guaiac was positive, leading one to believe that he suffers from intermittent gastrointestinal bleeding. Hepatic encephalopathy is another complication of chronic cirrhosis resulting from the portosystemic shunting of toxic gut contents (ammonia and other nitrogen based molecules, bacterial derived toxins) to the cerebral vasculature that can greatly alter mental status and possibly result in cerebral edema and herniation3. In fact the patient is being treated with the standard lactulose; a non-absorbable substrate that acidifies the GI lumen which has been found to be bactericidal to urease producing species4. As well he is being treated with rifaximin, a rifamycin antibiotic that inhibits bacterial RNA synthesis that has been found to have similar efficacy and faster onset4. Other manifestations of liver cirrhosis not covered here are hepatorenal and hepatopulmonary syndromes, hepatocellular carcinoma, and portal vein thromboses.
References:
1. Bosch J., Garcia-Tsoa G. “Management of Varices and Variceal Hemorrhage in Cirrhosis” N Engl J Med 2010 362:823-832.
2. Such J., Runyon B. “Pathogenesis of ascites in patients with cirrhosis” www.uptodate.com
3. Fauci, Braunwald, Kasper, Hauser, Longo, Jameson, Loscalzo. Harrison’s Principles of Internal Medicine 17th Edition. McGraw Hill 2008.
4. Zeneroli et al. “Management of Hepatic Encephalopathy: Role of Rifaximin.” Chemotherapy 2005 51:90-95.
5. Mansfield P. 2010. “Clinical features, diagnosis, and staging of gastric cancer” www.uptodate.com
6. Goldberg E., Chopra S., “Overview of the complications, prognosis, and management of cirrhosis” www.uptodate.com

No comments:
Post a Comment